This post was originally written by Nidhi Parekh for Fancy Comma LLC, and first appeared here.
With various countries cautiously emerging from lockdown, and social distancing still being enforced by governments, the hunt for a vaccine has never looked so desperate. In the United States, many states are rolling back reopening efforts due to a rise in new COVID-19 cases. In many states, bars and restaurants have closed dine-in operations to avoid sparking another drastic increase in COVID-19 infections.
Clearly, the clock is ticking for a solution to end the pandemic. Hundreds of vaccines are in the pipeline worldwide, but which will make it out of clinical trials and help us move past the COVID-19 pandemic?
To help you understand the race to a COVID-19 vaccine, Fancy Comma and The Shared Microscope have teamed up to profile promising vaccine candidates that are the frontrunners in this effort. This post is based on the information currently available through scientific papers, news articles, health news and updates, and existing scientific literature regarding vaccine development.
In a previous post, we explained how Moderna is leading the race with its cutting-edge mRNA-based vaccine. This post focuses on a close second: the vaccine currently in development by Oxford University and pharmaceutical giant AstraZeneca. Read on to learn about the origins of the Oxford vaccine, how it works, and why it is expected to be so promising in the global efforts to end the COVID-19 pandemic.
Could the Oxford/AstraZeneca vaccine speed up and overtake the development of the Moderna Vaccine? How does this vaccine work? Read on to learn the answers to these questions and more. @fancycomma
Could this vaccine speed up and overtake the development of the Moderna vaccine? How does the Oxford/Astrazeneca vaccine even work? Read on to learn the answers to these questions and more.
Better Together: Oxford University and AstraZeneca Team Up to Accelerate COVID-19 Vaccine Development
Just weeks after the SARS-CoV-2 vaccine was detected in Wuhan, China in December 2019, a team of Oxford scientists led by Dr. Sarah Gilbert got to work on designing a vaccine. On the 10th of January 2020, just weeks after the SARS-CoV-2 virus was detected in Wuhan, the Oxford team began their efforts to develop a vaccine. Since then, the Oxford team has identified a vaccine candidate, and completed Phase I trials. With the help of AstraZeneca, the team and has now entered Phase II/III trials in countries like Brazil and South Africa for its vaccine. AstraZeneca has partnered with the Oxford University team to accelerate development and large-scale vaccine production. Remember, for a vaccine to be developed and released to the public for use, it must pass all the necessary hurdles. This is to ensure safety and efficacy of the vaccine for use in large numbers.
How does the Oxford University/AstraZeneca vaccine work?
Curious about how vaccines work in general? Check out this post from The Shared Microscope on Instagram.
The Oxford/AstraZeneca vaccine uses a non-replicating viral vector to introduce vital information about a pathogen to the body of the host. But what information are we introducing, and what even is a viral vector? This is a bafflingly complex topic and to aid your understanding, we have simplified each part of it below.
The Oxford vaccine uses a non-replicating viral vector to introduce vital information about a pathogen to the body of the host @fancycomma #SciComm
Training Our Immune System to Defeat COVID-19
Like most of the vaccines being made against the novel coronavirus, the Oxford University vaccine, too, exploits the spike protein of the SARS-CoV-2 virus. The spike protein, as discussed in our earlier post, plays a very important role in the infection process. Without the spike protein, the SARS-CoV-2 virus cannot enter the host cell, where it typically makes more copies of itself. In other words, the spike protein is important for the virus’s ability to facilitate a COVID-19 infection. Neutralizing the effect of the spike protein, therefore, neutralizes the ability of the novel coronavirus to cause infection. The SARS-CoV-2 spike proteins are therefore a very important and potent therapeutic target. The Oxford/AstraZeneca vaccine trains our bodies to defend against the SARS-CoV-2 virus and prevent COVID-19 infection.
Most vaccines being developed against the novel coronavirus exploit the spike protein of the SARS-CoV-2 @fancycomma
What is a viral vector?
In biology, a viral vector is a modified version of a virus that is not only incapable of causing disease, but, when introduced into the body, can help us develop immunity to the virus. Viral vectors provide a convenient means to deliver antigens to the body. The antigens used in viral vector vaccines are weakened forms of bacteria or viruses. Once the vector introduces the body to an antigen, our body then develops immunity to the antigen.
Viral vectors are a convenient means to deliver weakened antigens to the body. @fancycomma
One analogy that can be used to explain viral vectors is that of “cut and paste.” We are all familiar with “cut and paste” — one might cut pictures out of a magazine and glue them together to produce decoupage art, or we may cut and paste text when we are working on our computers.
We can think of viral vectors as formed by selectively cutting certain genes which perform different but crucial functions, making various copies of these genes them — and adding them together to other genes which add new functions — to alter change the natural behaviour of the virus (and make it less infectious, for example). In biology, this cutting and pasting is called recombination. Different parts of viruses are combined together in new ways to create what is called a “recombinant” virus.
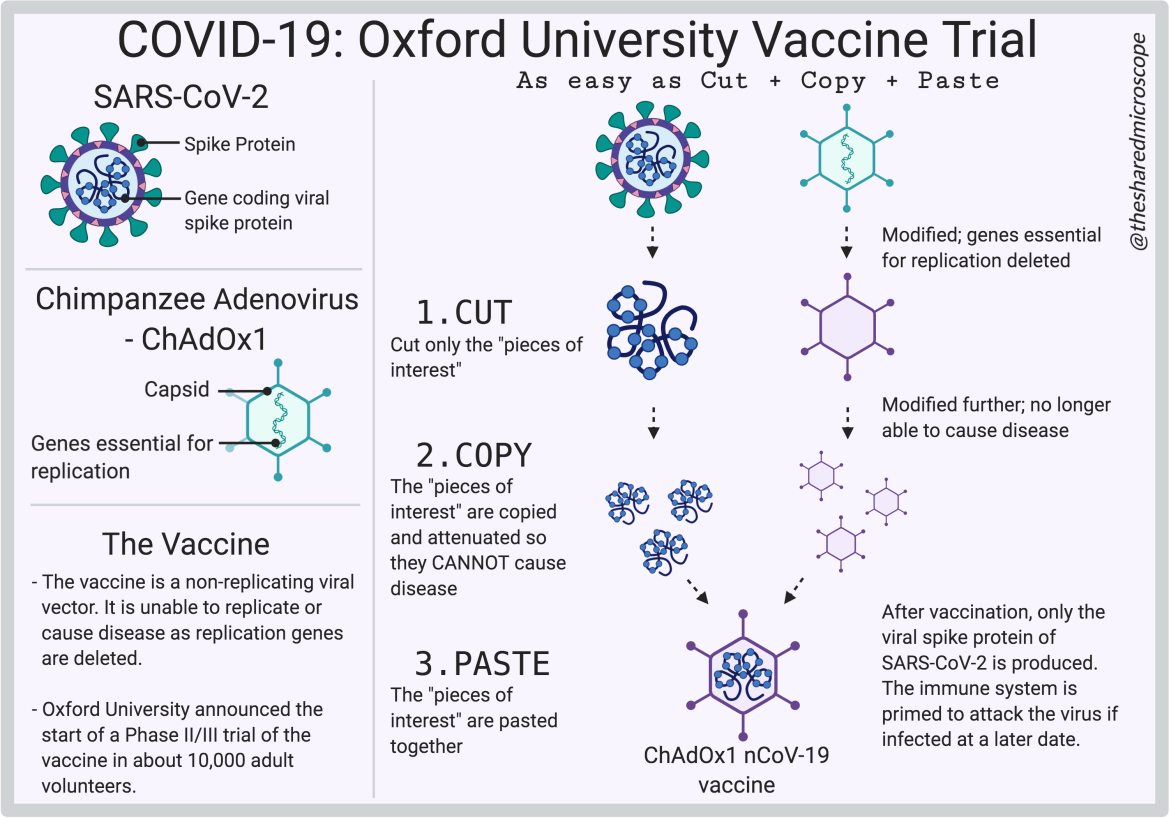
Caption: The Oxford/AstraZeneca Vaccine for COVID-19 uses a “Cut, Copy, Paste” Approach.
Illustration by The Shared Microscope made on Biorender.
Just take a virus, take out the parts that cause infection, add parts from another virus that do not cause infection (but do help you build immunity to that other virus), and voilà — you’ve got a viral vector which has some properties of both viruses, but is neither of those viruses. And to reiterate, cannot cause disease.
Just take a virus, take out the parts that cause infection, add parts from another virus that do not cause infection (but do help you build immunity to that other virus), and voilà — you’ve got a viral vector @fancycomma
Pasting Together Viruses to Make Vaccines
As we discussed in the previous section, cutting and pasting of different viruses is called recombination. The Oxford/AstraZeneca COVID-19 vaccine is a recombinant virus vaccine made of two different viruses. One of the viruses is SARS-CoV-2, the virus that causes COVID-19. The other virus in the recombinant vaccine is a chimpanzee adenovirus called ChAdOx1. Don’t worry if you don’t know anything about ChAdOx1 — we will discuss it more in this section.
The Oxford/AstraZeneca COVID-19 vaccine is a recombinant virus vaccine made from two different viruses – the SARS-CoV-2 and a chimpanzee adenovirus (ChAdOx1) @fancycomma
Normally, viruses are infectious, and the novel coronavirus is no exception. But with recombination, infectious viruses can be cut-and-pasted so that they are no longer infectious — the genetic material that is responsible for the virus’ infectious nature are simply cut out. Recombination allows scientists to create a non-infectious virus with characteristics that they desire from cutting-and-pasting different viruses together, as has been done with the Oxford/AstraZeneca vaccine.
In the recombinant viral vaccine developed by Oxford and AstraZeneca, the relevant parts of the two viruses are pasted together. This recombination is a frontrunner in the race to develop a COVID-19 vaccine.
Adenoviruses Are Commonly Used in Viral Vectors
One type of virus that is often used as a base for viral vectors are adenoviruses. Adenoviruses commonly infect people and cause mild symptoms similar to a cold or flu. This type of virus is also the basis for the viral vector used in the Oxford COVID-19 vaccine.
Although there are various kinds of adenoviruses, the Oxford University vaccine uses the Chimpanzee Adenovirus (ChAdOx1) as its base. Then, into this virus, the genetic material that encodes for the SARS-CoV-2 spike protein is added. The result is a virus that is not infectious but can cause the body to develop immunity to the spike protein and help stop the SARS-CoV-2 virus from entering and infecting cells.
The Oxford/AstraZeneca COVID-19 Vaccine Uses a Chimpanzee Adenovirus Called ChAdOx1
The basis for the Oxford/AstraZeneca COVID-19 vaccine is a chimpanzee adenovirus called ChAdOx1. Why did the Oxford researchers choose a chimpanzee adenovirus? The reason for this is simple. Human adenoviruses cause the common cold. These are viruses most of us will have some immunity to, as most of us are infected by these almost every year. So, if we were to use a human adenovirus as a viral vector, our immune system would destroy the human adenovirus as soon as it is detected by our body. The vector would therefore not work as effectively (if at all), as it would be destroyed before it could provide the host body (the human) with instructions on how to fight the novel coronavirus, which causes COVID-19.
Since humans aren’t exposed to the Chimpanzee Adenovirus (ChAdOx1) very often, its introduction would elicit an immune response in our cells. Inside this ChAdOx1 vector is the genetic information of only the SARS-CoV-2 spike protein, cut-and-pasted. The introduction of the ChAdOx1 would elicit an immune response in our cells. To ensure that the ChAdOx1cannot take hold of our system and make thousands of copies of itself, it is important that genes vital for its replication are “cut” out — deleted. What happens here, therefore, is that the virus will enter the human cell (singular) and infect it, but the disease is no longer able to spread. Each viral vector introduced to the body can only infect one cell, as opposed to infecting one cell and replicating to infect more.
With this ChAdOx1 vector (with the spike protein code cut-and-pasted inside), it would simply be the case that our bodies recognize the chimpanzee adenovirus as foreign, and then move to destroy it, and, in the process, become aware of the structure of the SARS-CoV-2 spike protein. As explained in our previous post, this is important because our body can then learn to identify these spike proteins, and then produce antibodies against it thereby neutralising it. Stopping/Neutralising the SARS-CoV-2 virus before it can infect the cell, is a very potent therapeutic target, and is being exploited in the production of a number of vaccines against COVID-19.
In normal infection scenarios, all of the genes essential for replication of the virus are intact i.e. they are not being deleted from the virus. In this case, the viruses are able to infect the cell, take over its machinery, and replicate by the dozen. These would then spread to other cells in the body and infect them too. It is when the viruses replicate and infect a number of other cells, that the disease is said to have manifested. More and more cells will therefore be infected. A good strategy is therefore to prevent it, by deleting the genes vital for replication of the vector virus.
So what is the ChAdOx1-nCov19 vaccine?
The ChAdOx1-nCov19 vaccine, developed by Oxford/AstraZeneca, is in essence a cut-and-paste version of both the chimpanzee adenovirus (ChAdOx1) and the SARS-CoV-2 virus. This vaccine uses the ChAdOx1 as a viral vector, and inserted into that, is the genetic information for the SARS-CoV-2 virus spike protein. For reiteration, the ChAdOx1 is unable to replicate, as some genes essential for replication have been deleted. So essentially, the Oxford/AstraZeneca vaccine looks like a ChAdOx1 virus on the outside, but its contents are that of the SARS-CoV-2 spike protein.
The genetic information to code for the spike protein is added to the ChAdOx1 vector. The viral vector (ChAdOx1) acts like a capsule inside which it carries its most prized possession — genetic information to produce the SARS-CoV-2 spike protein. The non-replicating vector will infect a single human cell, where it produces the antigen we want — the SARS-CoV-2 spike protein — to elicit an immune response.
Each viral vector that is introduced to the body can therefore only infect ONE cell. The adenovirus vector infection is unable to spread to all the cells in our body, because it cannot replicate. So, we do not become infected with the recombinant virus, and our body, in theory, learns to defend against both viruses — SARS-CoV-2, which causes COVID-19, and ChAdOx1. That way, it is thought, our bodies learn to resist COVID-19 infection.
What are the Advantages of a Viral Vector Vaccine?
A viral vector has a number of features that are particularly attractive for vaccine use. These advantages of viral vector vaccines include, but are not limited to:
- The ability to grow/produce the vaccine in a lab
- The inability of the vaccine to integrate into host genes
- Their stability
- Quick to make as compared to conventional vaccines (which can take on average 5-10 years), since it is based on part of the virus rather than the entire virus (more on this in our previous post)
- The ability to manufacture them in large scale under very controlled conditions
- Their inability to replicate and spread through the body, which means they can also be used in individuals who are immunocompromised
- Their ability to produce a very strong immune response – which means another “booster” dose may not be required
- Induction of both humoral and cell-mediated immune responses
What evidence is there to back up the ChAdOx1-nCov19 vaccine?
The Oxford Group has previously used the ChAdOx1 viral vector to protect against another coronavirus – the Middle East Respiratory Syndrome or MERS. SARS-CoV-2 has structural similarities with MERS. A similar type of spike protein is found on the MERS coronavirus (MERS-CoV), just as it is with SARS-CoV-2.
The MERS-CoV was initially a virus that causes cold-like symptoms in camels, but it jumped species and, because of the similarity of the entry site of cells in camels and humans, the MERS-CoV started to infect humans.
The gene that encodes the spike protein in MERS-CoV was then put into the ChAdOx1 vector and testing began at Oxford. Preclinical testing was carried out in animal models and Phase 1 clinical trials were also performed. The results of the animal studies have recently been published. The results of the Phase 1 clinical trials in humans were also published just yesterday.
The results of the Oxford studies showed that the antibodies against the MERS-CoV spike protein were higher in subjects injected with the ChAdOx1-MERS vaccine even a year after they were vaccinated. The immune response showed that the vaccine protected individuals and animal models from disease using both a cellular and humoral response (i.e. results showed an increase in T cells, and neutralising antibodies). For more on antibodies, please see our previous post. Based on this previous research done using MERS-CoV, it was possible to quickly discern what doses to use for the SARS-CoV-2 clinical trials.
Clinical Trials for the Oxford/AstraZeneca Vaccine
It took just a few months for the Oxford/AstraZeneca COVID-19 vaccine to go from preclinical trials in the laboratory on January 10, 2020 to human clinical trials on April 23, 2020.
For the ChAdOx1-nCov19 vaccine, preclinical studies were carried out on mice. The vaccine has the “nCoV19” as research began on this before the novel coronavirus was officially named SARS-CoV-2. In the study, mice were immunised using the ChAdOx1-nCov19 vaccine. Results showed that neutralising antibodies were produced in the mice, protecting them from infection when exposed to the SARS-CoV-2 virus.
Phase I trials of ChAdOx1-nCov19 vaccine started on April 23, 2020. 561 young and healthy individuals were vaccinated with the ChAdOx1-nCov19 vaccine, out of which 10 were vaccinated with 2 doses of the ChAdOx1-nCov19 vaccine. Another 551 were recruited for the control wing of the experiment, and were given licenced vaccines against meningitis. Since then, the Phase II/III trials also began. Here, the age range of people on which studies were carried out was expanded to include older people and children, so as to further study efficacy and safety (for more on vaccine development, feel free to visit our previous post). The study is still ongoing and therefore the results are currently unknown.
The vaccine has since been licensed to AstraZeneca, who will help accelerate development and large-scale production. AstraZeneca has committed to supply Europe 400 million doses of the vaccine, using manufacturers from different countries. They have also said they will not be taking any profits during the pandemic, and will support the ongoing clinical trials in Brazil and South Africa. AstraZeneca have also begun manufacturing at risk, without knowing whether the vaccine is effective against SARS-CoV-2 in humans. The World Health Organization (WHO) believes that this vaccine is the contender in the race to find the COVID-19 vaccine, and end this pandemic.
For more on the Oxford / AstraZeneca vaccine, please check out this YouTube video:
Related posts:
 Covid-19: Let’s Get Handsy!
Covid-19: Let’s Get Handsy!
 [Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing September 28, 2020
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing September 28, 2020
 [Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing November 09, 2020
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing November 09, 2020
 [Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing November 16, 2020
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing November 16, 2020
 [Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing January 18, 2021
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing January 18, 2021
- Written by: admin
- Posted on: July 3, 2020
- Category: COVID-19

0 comments
Louis Demaris
July 4, 2020 at 12:02 pmHello there! I just want to give you a huge thumbs up for your great information you have right here on this post. I will be returning to your site for more soon.
Nidhi
July 4, 2020 at 4:47 pmThank you so much for saying that! We are working behind the scenes to make more illustrations and also write more content, not only on the COVID-19 vaccines but also other interesting science topics. If you like what you see/read, make sure to subscribe. We are also on Facebook as The Shared Microscope, on Instagram @thesharedmicroscope and tweeting @thesharedscope. Please also share with friends and family you think would be interested.
learn more
July 31, 2020 at 5:55 amThis excellent website truly has all of the information and facts I wanted concerning this subjectand didn’t know who to ask.
Novavax: A SARS-CoV-2 “Protein Factory” to Beat COVID-19 – The Shared Microscope
August 3, 2020 at 10:47 am[…] Oxford/AstraZeneca vaccine based on viral […]
Vaccine Development: A Chronology – The Shared Microscope
August 3, 2020 at 5:30 pm[…] a look at some of our previous posts. We have previously written posts on the Moderna vaccine, the Oxford/AstraZeneca vaccine, the CoronaVac developed by Sinovac, and the vaccine developed by […]
CoronaVac: A COVID-19 Vaccine Made From Inactivated SARS-CoV-2 Virus – The Shared Microscope
August 19, 2020 at 6:18 pm[…] way in this international race to a vaccine for the novel coronavirus are the United Kingdom’s Oxford/AstraZeneca vaccine and the United States’ Moderna […]
Why the Anti-Vax Rhetoric Prevails – The Shared Microscope
September 1, 2020 at 6:59 pm[…] types of vaccines and more specifically how some of the COVID-19 vaccines (the Moderna vaccine, Oxford University/AstraZeneca Vaccine, the vaccine in development by Sinovac, and the Novavax vaccine) in development […]
Lemuel Gibert
September 11, 2020 at 5:09 pmThis is good insight for my crowd, so I’ll share this post and you should likely get a few extra readers. It’s a step up from anything else I’ve seen discussing this subject. Thank you for the inspired viewpoint!
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing September 07, 2020 – The Shared Microscope
September 13, 2020 at 1:23 am[…] […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing September 14, 2020 – The Shared Microscope
September 20, 2020 at 6:44 pm[…] […]
Pfizer and BioNTech’s mRNA Vaccine Against COVID-19: How Does It Work? – The Shared Microscope
September 23, 2020 at 2:41 pm[…] to develop a COVID-19 vaccine in a timely manner. This vaccine, like the Moderna vaccine, the Oxford University/AstraZeneca vaccine, the vaccines in development by Novavax and Sinovac, is another frontrunner in this race […]
lose weight fast
September 29, 2020 at 3:42 pmThis is inspired! I’m glad I read your post as it’s an improvement on similar blogs I’ve seen from most other bloggers on this subject. Is it okay if I ask you to write more about this? Could you provide another example? Thanks 🙂
Tiana Griego
October 23, 2020 at 11:10 amYes, this is a Good one
Johnson and Johnson’s COVID-19 Vaccine: How Does It Work? – The Shared Microscope
December 1, 2020 at 12:48 am[…] of two viruses. Just how does a viral vector vaccine work? As we wrote in our post about the Oxford/AstraZeneca vaccine, “Just take a virus, take out the parts that cause infection, add parts from another virus that […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing December 07, 2020 – The Shared Microscope
December 14, 2020 at 8:37 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing November 30, 2020 – The Shared Microscope
December 14, 2020 at 8:39 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing November 23, 2020 – The Shared Microscope
December 14, 2020 at 8:41 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing December 14, 2020 – The Shared Microscope
December 21, 2020 at 9:08 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing December 21, 2020 – The Shared Microscope
December 28, 2020 at 6:04 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing December 28, 2020 – The Shared Microscope
January 4, 2021 at 7:49 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing January 04, 2020 – The Shared Microscope
January 11, 2021 at 6:55 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing January 11, 2021 – The Shared Microscope
January 18, 2021 at 4:22 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing January 18, 2021 – The Shared Microscope
January 25, 2021 at 8:01 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing January 25, 2021 – The Shared Microscope
February 1, 2021 at 5:48 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing February 01, 2021 – The Shared Microscope
February 8, 2021 at 8:56 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing February 08, 2021 – The Shared Microscope
February 15, 2021 at 7:32 pm[…] 1. The Oxford/AstraZeneca Vaccine […]
[Newsletter] Round-Up of COVID-19 Vaccine Updates: Week Commencing January 04, 2021 – The Shared Microscope
August 1, 2021 at 10:45 pm[…] 1. The Oxford/AstraZeneca Vaccine […]